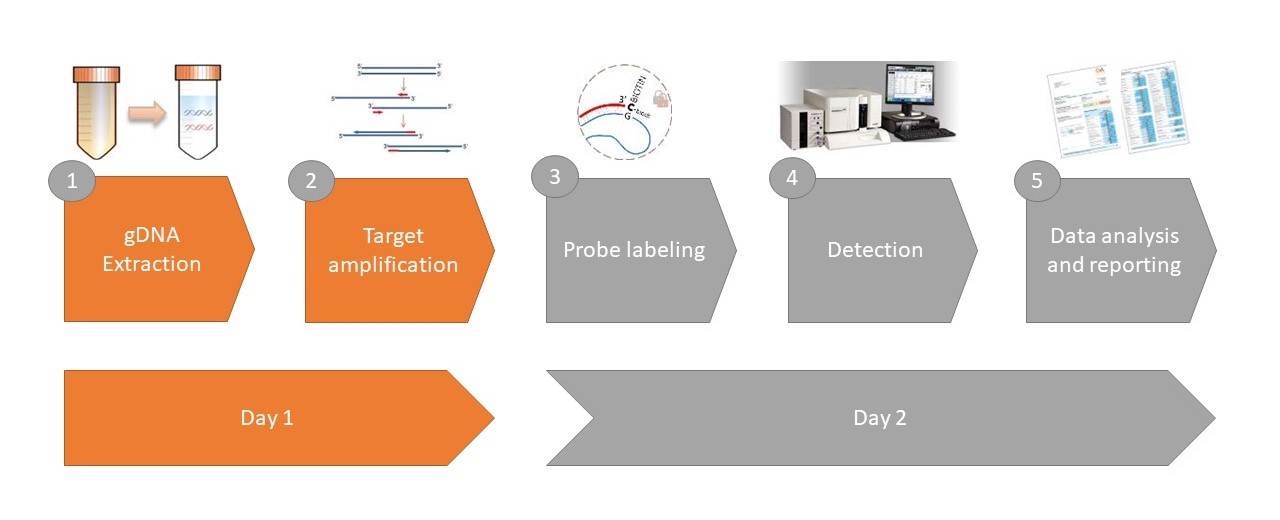
The Eagle Bioscience’s Free Soluble RANKL ELISA was utilized in a recent publication focusing on Skeletal Disease acquisition in Fibrous Dysplasia. Check out the full text and abstract below.
Abstract
Fibrous dysplasia (FD) is a rare mosaic disorder resulting in fractures, pain, and disability. Bone lesions appear during childhood and expand during skeletal growth. The rate at which FD lesions progress and the biochemical determinants of FD lesion formation have not been established, making it difficult to investigate and implement preventative therapies. The purpose of this study was to characterize FD lesion progression in children, and to identify clinical variables associated with progressive disease. Clinical data and imaging from an ongoing natural history study at the National Institutes of Health (NIH) were reviewed. 99m-Technetium methylene diphosphonate (99Tc-MDP) scans were used to determine Skeletal Burden Score (SBS), a validated quantitative scoring system. FD progression rate was determined by the change in the SBS in each patient per year. Thirty-one children had serial 99Tc-MDP scans, with a median age at first scan of 6 years (interquartile range [IQR] 4–8, range 2–10), and median follow-up 1.1 years (IQR 1.1–2.1, range 0.7–11.2). The median FD progression rate for the total group was 2.12 SBS units/year (IQR 0.81–2.94, range 0.05–7.81). FD progression rates were highest in children under age 8 years and declined with age (p = 0.03). Baseline disease severity was associated with subsequent disease progression (p = 0.009), with the highest FD progression rates in patients with moderate disease (baseline SBS 16–30), and lowest progression rates in those with severe disease (SBS ≥50). Serum levels of the bone formation marker osteocalcin were positively correlated with subsequent FD progression rate (p = 0.01, R = 0.58). There was no association between FD progression and baseline endocrinopathies, fractures, or surgery rates. FD lesions progress during childhood, particularly in younger children and those with moderate involvement. Osteocalcin may potentially serve as a biomarker for progressive disease. These findings may allow clinicians to investigate preventative therapies, and to identify children with FD who are candidates for early interventions. Published 2022. This article is a U.S. Government work and is in the public domain in the USA.
If you have any questions about the Free Soluble RANKL ELISA or our other offerings, please contact us here.