The Biomedica Human IL-6 High Sensitive ELISA Assay Kit was highlighted in a recent publication that explored factors associated with incident vertebral fractures in glucocorticoid-treated Duchenne muscular dystrophy. Check out the abstract and full text below!
Abstract
Purpose: Prevention of fractures is an unmet need in glucocorticoid (GC)-treated Duchenne muscular dystrophy. This study explored factors associated with incident vertebral fractures (VFs) to inform future fracture prevention efforts.
Methods: VFs were evaluated prospectively at study baseline and 12 months on lateral spine radiographs in participants aged 4 to 25 years with Duchenne muscular dystrophy. Clinical factors were analyzed for their association with the change in Spinal Deformity Index (sum of the Genant-defined VF grades from T4 to L4) between baseline and 12 months.
Results: Thirty-eight males were evaluated (mean ± SD age at baseline 11.0 ± 3.6 years; mean ± SD GC duration at baseline 4.1 ± 3.1 years; 74% ambulatory). Nine of 38 participants (24%) had 17 incident VFs, of which 3/17 VFs (18%) were moderate/severe. Participants with 12-month incident VF had lower mean ± SD baseline lumbar spine areal bone mineral density Z-scores (-2.9 ± 1.0 vs -1.9 ± 1.1; P = .049) and lower total body less head areal bone mineral density Z-scores (-3.1 ± 1.2 vs -1.6 ± 1.7; P = .036). Multivariable linear regression showed that at least 1 VF at baseline (P < .001), a higher number of antecedent non-VF (P < .001), and greater bone age delay at baseline (P = .027) were significant predictors of an increase in the Spinal Deformity Index from baseline to 12 months.
Conclusion: The observation that ≥ 1 prevalent VF and/or non-VF were the strongest predictors of incident VFs at 12 months supports the need for prevention of first fractures in this high-risk setting. Bone age delay, a marker of GC exposure, may assist in the prioritization of patients in efforts to prevent first fractures.
Keywords: Duchenne muscular dystrophy; bone fragility; glucocorticoids; incident fractures; osteoporosis; vertebral fractures.
If you have any questions about this product or any of our other offerings, contact us here.






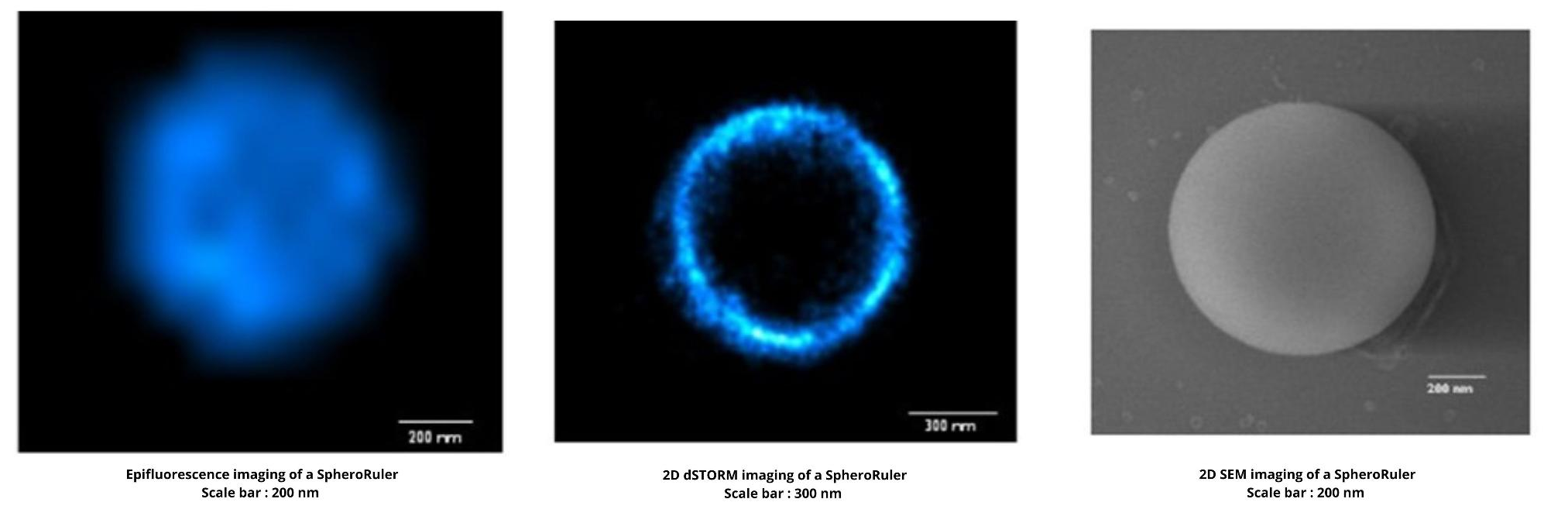
 What is it SpheroRuler intended for?
What is it SpheroRuler intended for?