
Eagle Biosciences will be at AACC in Anaheim, California!
This year AACC is in Anaheim at the Anaheim Convention Center, Sunday July 23th – Thursday July 27th. We will be at booth #1147 from July 25th to July 27th! Come by to learn about the GA-Map Dybiosis Test Lx and more assays that could help you with your microbiome or other research! We will be there to answer any questions you may have, or just stop by and say hi! We love seeing our customers!
Product Highlights
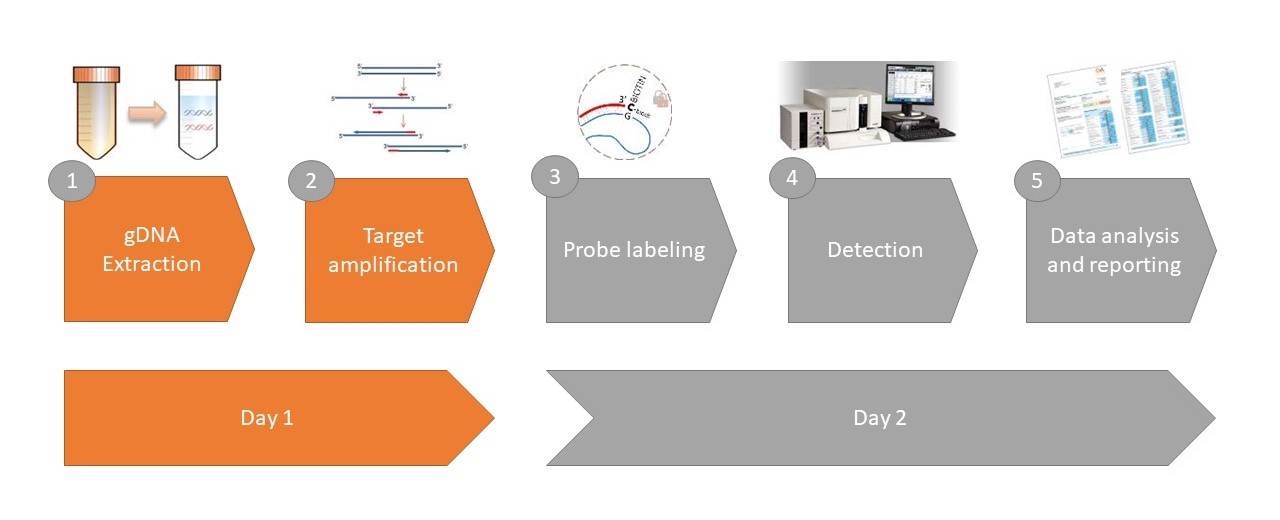
GA-Map Dysbiosis Test Lx: The first and only standardized solution for microbiome profiling! The GA-Map Dysbiosis Test Lx is a simple multiplex stool assay that maps the intestinal microbiota profile for a selected set of bacteria. The GA-map® platform uses probes that target variable regions (V3 to V7) of the bacterial 16S rRNA gene to characterize and identify bacteria present. The targets are identified in a molecular multiplex assay that utilizes the Single Nucleotide Primer Extension (SNuPE) technology patented by Professor Knut Rudi (US6617138). A unique algorithm takes advantage of all the data generated by the detection of the SnuPE products to determine dysbiosis level in the sample. The algorithm is incorporated in the GA-map® Dysbiosis Analyzer software that accompanies the test.
GA-map® Dysbiosis Test Lx Procedure Quick Guide
If you have any other questions about these products or our other offerings, contact us here.

