
What is Vanin-1?
Vanin-1 is an epithelial ectoenzyme activating the conversion of pantetheine into pantothenic acid (vitamin B5) and cysteamine and thus is involved in the regulation of oxidative stress and inflammation.
Why is Vanin-1 important?
The highest levels of Vanin-1 expression is assigned to renal tubular epithelial cells. Hence, Vanin-1 released from renal cells can be detected in the urine. Pre-clinical and clinical studies demonstrate that Vanin-1 is an early biomarker of renal tubular damage in drug-induced acute kidney injury, obstructive nephropathy, and diabetic nephropathy. In fact, Vanin-1 has as superior predictive value for acute kidney injury than established markers KIM-1, NGAL, or NAG .
Comparison of human urine Vanin-1 values in apparently healthy individuals and individuals with kidney disease:
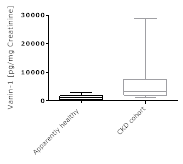
Areas of Interest:
- Acute kidney injury
- Diabetic nephropathy
- Drug-induced acute kidney injury
- Obstructive nephropathy
Assay Highlights:
- Optimized for human urine samples
- Highly SPECIFIC and DEFINED characterized antibodies
- RELIABLE rigorously validated according to FDA guidelines
- QUICK one-step ELISA
Eagle Biosciences offers the first fully validated human Vanin-1 assays in a simple-to-use ELISA:
Human Vanin-1 (urine) ELISA Assay Kit
Contact us for more information about our Vanin-1 related assay kits.
Eagle Biosciences will be at ASN Kidney Week in Washington, DC!
Stop by booth #2005 to learn more about Vanin-1, Klotho, Intact FGF23 and other assays that could help you with your kidney related research! We’ll be there to answer any questions you may have, or stop and say hi! We love seeing our customers!






