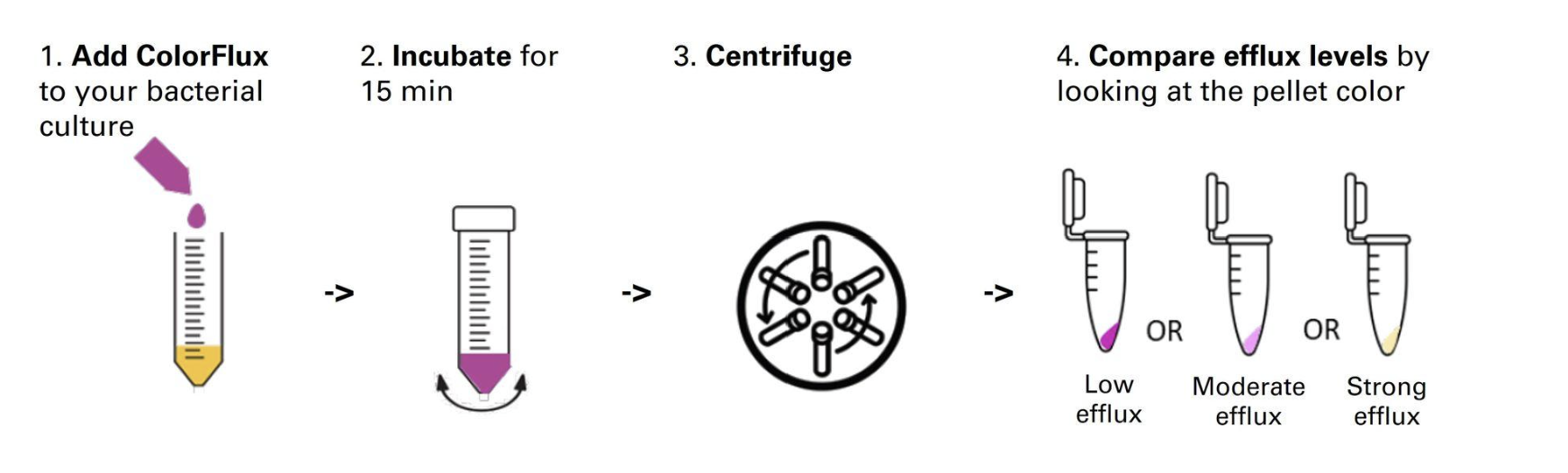
What is it intended for?
DNAbsolute is composed of a ionic liquid that has a selective and high affinity for DNA strands. When mixed with your sample, the DNAbsolute reagent will directly bind DNA and precipitate it without the need for RNase treatment, protein precipitation or use of hazardous reagents.
DNAbsolute has been successfully used for extracting DNA from insects (drosophila, psyllids, beetles, ants, cochineals), plants (dried leaves) and bacteria (Salmonella). Once your sample is lysed, the whole DNA extraction process should take no more than 30 minutes.
Technical information
Bacterial species compatibility : Gram +
Tested and validated on : Staphylococcus aureus, Bacillus subtilis, Streptococcus pneumoniae.
Colour: Red
Form: liquid
Detection methods: visual & fluorescence. Also compatible with single-cell approaches (live imaging and flow cytometry)
Peak excitation/emission wavelengths: Ex:530nm/Em:650nm
Storage: at 4°C protected from light for 8 months
1 kit contains 1mL of an aqueous solution with a concentration of 1 mg/mL for a final volume of 1L of screening medium
Features of DNAbsolute
- Safe & Simple – No need for any columns or harmful chemicals, highly amenable to automated workflows & on-field applications
- Versatile – Extract DNA from a variety of samples, including some of the most challenging ones (i.e. insects, plants & corals)
- Extreme Sensitivity – Efficient DNA recovery from reduced starting materials, including individual specimens and even down to a single leg!
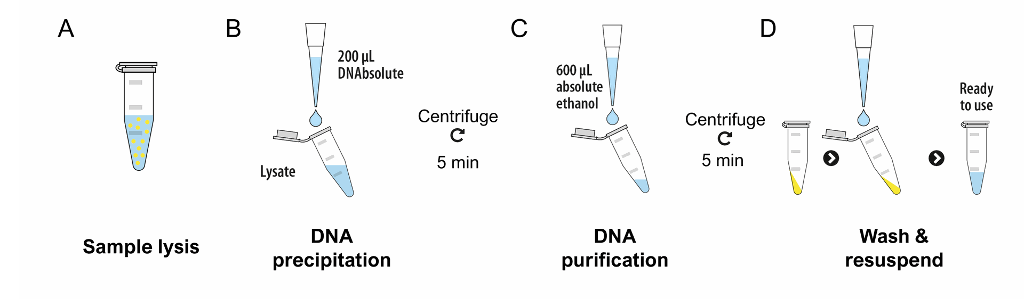
Simplified Protocol
Frequently Asked Questions
What yield can I expect?
The resulting yield can vary widely depending on the sample type and preparation. As a general rule, you can expect to recover on average 85% of DNA from a solution ranging from 10 to 100 μg/mL of DNA, and 60% of a solution ranging from 5 to 10 ng/mL of DNA. Poor quality, and/or fragmented starting material will result in reduced yield of purified DNA.
How pure will the extracted DNA be?
DNAbsolute DNA extraction method can yield highly pure DNA due to its ability to efficiently and specifically solubilize DNA, minimizing contamination with proteins, RNA, and other cellular debris. The purity of drosophila DNA extracted using DNAbsolute has been assessed by spectroscopic measurements. The average 260/280 ratio was found between 1.8-1.9 and the 260/230 ratio at 2.3.
The type of biological sample being processed can affect the extracted DNA purity. Sample lysis optimization, or additional purification steps, might be implemented when working with samples containing high levels of secondary metabolites.
For more information about this product or any others from the Microscopy line, contact us here.