Rheumatoid Factor IgG ELISA Kit
Rheumatoid Factor IgG ELISA Kit Developed and Manufactured by Medipan
Size: 1×96 wells
Sensitivity: 1 U/ml
Dynamic Range: 1 – 300 U/ml
Incubation Time: 2.5 hours
Sample Type: Serum, Plasma
Sample Size: 10 µL
Alternative Names: Rheumatoid Factor IgG ELISA, Human RF IgG ELISA, RF IgG
For Research Use Only
Calibration
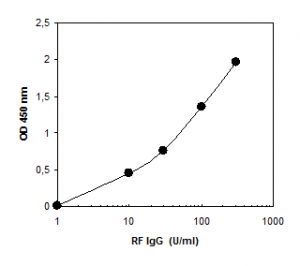
Rheumatoid Factor IgG is calibrated in arbitrary units U/ml.
Linearity
Dilutions of selected positive specimens in Rheumatoid Factor IgG autoantibody free human serum are determined according to their expected theoretical values with RF IgG.
Assay Principle for Rheumatoid Factor IgG ELISA
The Rheumatoid Factor IgG ELISA Kit is an enzyme immunoassay for the quantitative determination of IgG antibodies to the Fc region of IgG in human serum or plasma. The rheumatoid factors of the calibrators, control and diluted patient samples react with rabbit IgG immobilized on the solid phase of microtiter plates. Following an incubation period of 60 min at room temperature, unbound sample components are removed by a wash step. The bound IgG antibodies react specifically with anti-human-IgG conjugated to horseradish peroxidase (HRP). Within the incubation period of 30 min at RT, excessive conjugate is separated from the solid-phase immune complexes by the following wash step. HRP converts the colorless substrate solution of 3,3’,5,5’-tetramethyl¬benzidine (TMB) added into a blue product. The enzyme reaction is stopped by dispensing an acidic solution into the wells after 15 min at room temperature turning the solution from blue to yellow. The optical density (OD) of the solution at 450 nm is directly proportional to the amount of specific antibodies bound. The standard curve is established by plotting the antibody concentrations of the calibrators (x-axis) and their corresponding OD values (y-axis) measured. The concentration of antibodies of the specimen is directly read off the standard curve.