Golimumab mAb-based ELISA Assay
The Golimumab mAb-based ELISA Assay is For Research Use Only
Size: 1 x 96 wells
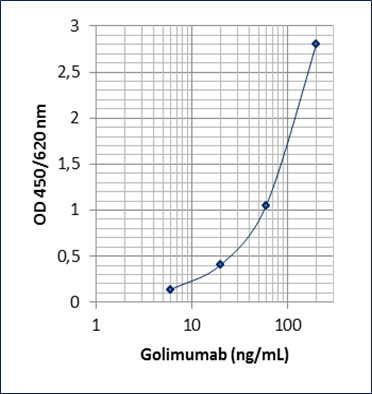
Sensitivity: 5 ng/mL
Dynamic Range: 6 – 200 ng/mL
Incubation Time: 2 hours
Sample Type: Serum, Plasma
Sample Size: 10 µL
Alternative Names: Simponi
Assay Background
The drug Golimumab (trade name Simponi®) is a human monoclonal antibody that binds to both the soluble and transmembrane bioactive forms of human TNFα. This interaction prevents the binding of TNFα to its receptors, thereby inhibiting the biological activity of TNF. Golimumab has been proven effective in the treatment of Rheumatoid Arthritis (RA), Ankylosing Spondylitis (AS), Psoriatic Arthritis (PsA) or Ulcerative Colitis (UC). Antibodies to Golimumab were detected in 57 (4%) of Golimumab-treated patients across the Phase 3 RA, PsA, and AS trials through Week 24. Similar rates were observed in each of the three indications. Patients who received Golimumab with concomitant Methotrexate (MTX) had a lower proportion of antibodies to Golimumab than patients who received Golimumab without MTX (approximately 2% versus 7%, respectively). Of the patients with a positive antibody response to Golimumab in the Phase 2 and 3 trials, most were determined to have neutralizing antibodies to Golimumab as measured by a cell-based functional assay. The data from the literature demonstrated that Anti-Drug Antibody positivity was significantly associated with low Golimumab levels and poor therapeutic response.
This ImmunoGuide Golimumab ELISA (mAb-based) is developed for the specific measurement of Golimumab in sera, plasma and other biological fluids by the advantage of using a site-directed DWZ-1E4 mouse monoclonal antibody (mAb) specific for Golimumab only.