
Eagle Bioscience’s is excited to highlight the Alpha Synuclein Preformed Fibrils (Type 1) from StressMarq!
About the Alpha Synuclein Pre-Formed Fibrils (Type 1)
StressMarq’s Alpha Synuclein Pre-formed Fibrils (Type 1) are produced with low endotoxin levels, making them the suitable choice for both in-vivo and in-vitro work. Alpha Synuclein PFFs seed the formation of new fibrils from active alpha synuclein monomers, and can be used to induce endogenous alpha synuclein phosphorylation and subsequent Lewy body inclusion formation in neuronal cell culture or for in vitro oligomerization studies.
Background
Alpha-Synuclein (SNCA) is expressed predominantly in the brain, where it is concentrated in presynaptic nerve terminals1. Alpha-synuclein is highly expressed in the mitochondria of the olfactory bulb, hippocampus, striatum and thalamus2. Functionally, it has been shown to significantly interact with tubulin3, and may serve as a potential microtubule-associated protein. It has also been found to be essential for normal development of the cognitive functions; inactivation may lead to impaired spatial learning and working memory4. SNCA fibrillar aggregates represent the major non A-beta component of Alzheimers disease amyloid plaque, and a major component of Lewy body inclusions, and Parkinson’s disease. Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by the progressive accumulation in selected neurons of protein inclusions containing alpha-synuclein and ubiquitin5, 6.
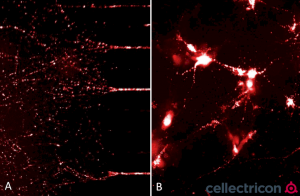
ATTO633 fluorescently-labelled alpha synuclein PFFs (SPR-322) were taken up, transported into the soma, and induced alpha synuclein aggregation in mouse neurocortical primary cells. (A) Neurites filled with fluorescently-labelled alpha synuclein seeds in a microfluidic co-culture system after 24 hours. (B) Alpha synuclein seeds within the soma and neurites of mouse neurocortical primary cells after 24 hours. Experiment and imaging courtesy of Cellectricon.
References
- “Genetics Home Reference: SNCA”. US National Library of Medicine. (2013).
- Zhang L., et al. (2008) Brain Res. 1244: 40-52.
- Alim M.A., et al. (2002) J Biol Chem. 277(3): 2112-2117.
- Kokhan V.S., Afanasyeva M.A., Van’kin G. (2012) Behav. Brain. Res. 231(1): 226-230.
- Spillantini M.G., et al. (1997) Nature. 388(6645): 839-840.
- Mezey E., et al. (1998) Nat Med. 4(7): 755-757.
The Alpha Synuclein Preformed Fibrils (Type 1) were utilized in the following studies:
- Deciphering the role of hsp110 chaperones in diseases of protein misfolding. Yakubu, U. M. (2021) PhD Thesis, University of Texas.
- Suppression of aggregate and amyloid formation by a novel intrinsically disordered region in metazoan Hsp110 chaperones. Yakubu, U.M., Morano, K.A. (2021) J Biol Chem Jan-Jun 2021;296:100567.
- Design, Synthesis and Chemically Engineered Graphene Quantum Dot Applications: Contrast Agent for MR Imaging and Targeted Therapeutics on Parkinson’s Treatment. Poonkuzhali, K. et al. (2022) SSRN 4056733.
- Rational Generation of Monoclonal Antibodies Selective for Pathogenic Forms of Alpha-Synuclein. Gibbs, E. et al. (2022) Biomedicines 10,2168.
- LRP1 is a neuronal receptor for α-synuclein uptake and spread. Chen, K. et al. (2022) Mol Neurodegener 17(1):57.
- Granulovacuolar degeneration bodies are independently induced by tau and α-synuclein pathology. Jorge-Oliva, M. et al. (2022) Alzheimer’s Research and Therapy (14)187.
If you have any questions about this product or any of our other offerings, contact us here.
