Omalizumab mAb-Based ELISA Assay
The Omalizumab mAb-Based ELISA Assay is For Research Use Only
Size: 1 x 96 wells
Sensitivity: 1 ng/mL
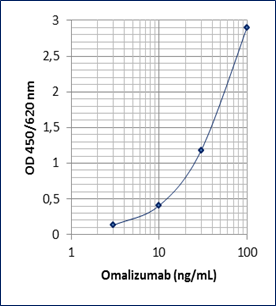
Dynamic Range: 3 – 100 ng/mL
Incubation Time: 2 hours
Sample Type: Serum, Plasma
Sample Size: 10 µL
Alternative Name: Xolair
Assay Background
Omalizumab (trade name Xolair®) is a recombinant DNA-derived humanized IgG1κ monoclonal antibody and it binds to human immunoglobulin E (IgE). The molecular weight of omalizumab is 149 kilodlatons and is produced by Chinese hamster ovary cell suspension culture. Omalizumab inhibits the binding of IgE to IgE receptor (FcεRI) on the surface of mast cells and basophils. Therefore, the Omalizumab is expected to limit the degree of release of mediators of the allergic response from the FcεRI bearing cells.
This ImmunoGuide Omalizumab ELISA (mAb-based) is developed for the specific measurement of Omalizumab in serum, plasma and other biological fluids by the advantage of using a site-directed IG-Ulkr4H1 mouse monoclonal antibody (mAb) specific for Omalizumab only. Binding of Omalizumab to the solid phase, pre-coated with IG-Ulkr4H1, is inhibited by human IgE in a concentration dependent manner. Therefore, the ImmunoGuide Omalizumab ELISA (mAb-based) measures the free form of Omalizumab only. The choice of specifically measuring the free form allows investigators to analyze the concentration-effect relationship. The ImmunoGuide Omalizumab ELISA (mAb-based) kit can be efficiently used for measuring free Omalizumab levels in serum and plasma.
STORAGE AND STABILITY OF THE KIT
The kit is shipped at ambient temperature and should be stored at 2-8°C. Keep away from heat or direct sun light. The microtiter strips are stable up to the expiry date of the kit in the broken, but tightly closed bag when stored at 2–8°C.