Sclerostin ELISA Assay Kit
Sclerostin ELISA Assay Kit Developed and Manufactured in Austria by Biomedica
Size: 1×96 wells
Sensitivity: LOD: (0 pmol/l + 3 SD): 3.2 pmol/l; LLOQ: <7.5 pmol/l
Dynamic Range: 0 to 240 pmol/l
Incubation Time: > 24 hours
Sample Type: Serum, Plasma (EDTA, Heparin), Urine protocol available
Sample Size: 20 µL
Alternative Names: SOST,CDD, VBCH, DAND6, SOST1
For Research Use Only
Controls Included
Product Developed and Manufactured in Austria by Biomedica
Unit conversion: 1 pg/ml = 0.044 pmol/l (MW: 22.5 kD)
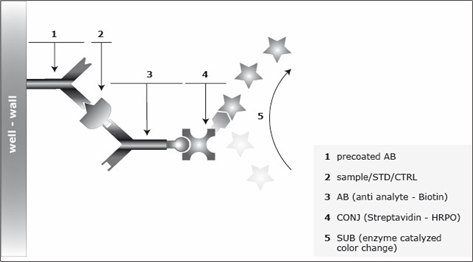
Assay Principle for the Sclerostin Assay
The Sclerostin ELISA Assay Kit is a sandwich enzyme immunoassay for the quantitative determination of Sclerostin (SOST) in human serum, plasma, urine and cell culture supernatnat samples. In a first step, assay buffer is pipetted into the wells of the microtiter strips. Thereafter, standard/control/sample and detection antibody (biotinylated monoclonal mouse anti-human Sclerostin) are pipetted into the wells, which are pre-coated with polyclonal goat anti-human Sclerostin antibody. Any Sclerostin present in the standard/control/sample binds to the pre-coated antibody in the well and forms a sandwich with the detection antibody. In a washing step, all non-specific unbound material is removed. In a next step, the conjugate (strepdavidin-HRP) is pipetted into the wells and reacts with the detection antibody. After another washing step, the substrate (tetramethylbenzidine, TMB) is pipetted into the wells. The enzyme-catalyzed color reaction of the substrate is directly proportional to the amount of Sclerostin present in the sample. This color change is detectable with a standard microtiter plate reader. A dose response curve of the absorbance (optical density, OD, at 450 nm) versus standard concentration is generated, using the values obtained from the standards. The concentration of human Sclerostin in the sample is determined directly from the dose response curve. The human Sclerostin (SOST) immunoassay is an overnight, 96-well sandwich ELISA for the quantitative determination of human Sclerostin in serum, plasma, urine and cell culture supernatant. The assay employs human serum-based standards to ensure the measurement of biologically reliable data. The Sclerostin (SOST) ELISA kit uses highly purified, epitope mapped antibodies.
Related Products
Bioactive Sclerostin ELISA Kit
Soluble Semaphorin 4D ELISA
Osteoprotegerin ELISA Assay Kit