MMAE ADC ELISA Kit
MMAE ADC ELISA Kit Developed and Manufactured in the USA
Size: 1×96 wells
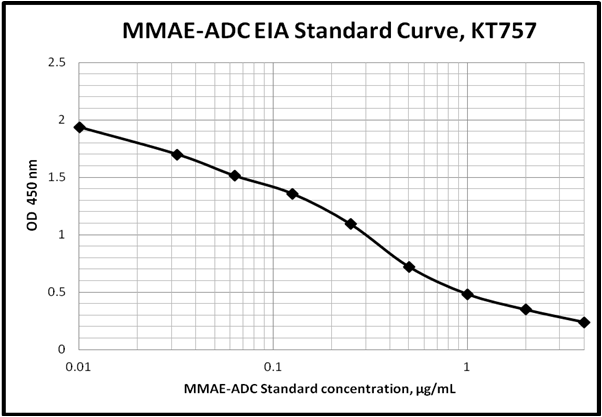
Sensitivity: 0.0435 µg/mL
Dynamic Range: 0.032 – 4.0 µg/mL
Incubation Time: 2.5 hours
Sample Type: Serum, Cell Culture, Tissue
Sample Size: 100 µL
Alternative Names: Monomethyl auristatin E, MMAE Antibody Drug Conjugate ELISA Kit
For Research Use Only
Controls Included
Assay Principle
The MMAE ADC ELISA Kit is designed, developed and produced for the quantitative measurement of antibody Monomethyl auristatin E (MMAE) conjugate in serum. The assay utilizes the competitive immunoassay technique with an antibody that exclusively binds to Monomethyl auristatin E.
Assay calibrators (antibody MMAE conjugate) and test serum samples are added directly to wells of a microtiter plate that is coated with specific anti-MMAE antibody. Subsequently, a horseradish peroxidase (HRP) conjugated MMAE is added to each well. During the incubation period, the antibody MMAE conjugate competes with the HRP conjugated MMAE for the limited binding sites of anti-MMAE antibody. An immune complex of well coated “anti-MMAE antibody – HRP conjugated MMAE” is formed. The unbound antibodies and buffer matrix are removed in the subsequent washing step. For the detection of this immunocomplex, the well is then incubated with a substrate solution in a timed reaction, which is terminated with an acidic reagent (i.e. ELISA stop solution). The absorbance is then measured in a spectrophotometric microplate reader. The enzymatic activity of the immunocomplex bound to the wall of each microtiter well is inversely proportional to the amount of antibody-MMAE conjugate in the test sample. A calibration curve is generated by plotting the absorbance versus the respective antibody-MMAE conjugate concentration for each calibrator on a 4-parameter or log-logit curve fitting. The concentration of antibody-MMAE conjugate in test samples is determined directly from this calibration curve.