Etanercept mAb-based ELISA Assay
The Etanercept mAb-based ELISA Assay is For Research Use Only
Size: 1 x 96 wells
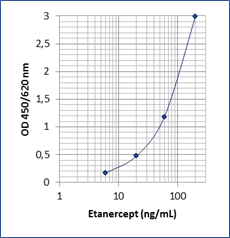
Sensitivity: 5 ng/mL
Dynamic Range: 6 – 200 ng/mL
Incubation Time: 2 hours
Sample Type: Serum, Plasma
Sample Size: 10 µL
Alternative Names: Enbrel Assay
Assay Principle
This Etanercept ELISA is based on Etanercept-specific mouse monoclonal antibody (catcher Ab, ImmunoGuide clone 5A1). Diluted standards and samples are incubated in the microtiter plate coated with IG-5A1 mAb. After incubation, the wells are washed. A horseradish peroxidase (HRP)-conjugated anti-human IgG monoclonal antibody is added and binds to the Fc part of Etanercept. Following incubation, wells are washed and the bound enzymatic activity is detected by addition of chromogen-substrate. The color developed is proportional to the amount of Etanercept in the sample or standard. Results of samples can be determined by using the standard curve. Binding of Etanercept to the solid phase, pre-coated with 5A1, is inhibited by recombinant human TNF-alpha in a concentration dependent manner. Therefore, the ImmunoGuide Etanercept ELISA (mAb-based) measures the free form of Etanercept. Pipette 100µL of Assay Buffer into each of the wells to be used.
STORAGE AND STABILITY OF THE KIT
The kit is shipped at ambient temperature and should be stored at 2-8°C. Keep away from heat or direct sun light. The microtiter strips are stable up to the expiry date of the kit in the broken, but tightly closed bag when stored at 2–8°C.