GA-map® Dysbiosis Test Lx
GA-map Dysbiosis Test Lx is Developed and Manufactured in Norway by Genetic Analysis
The GA-map Dysbiosis Test Lx contains:
- Box A: reagents for PCR, end-labeling reaction, and control material
- Box B: GA-map® Bead Set, GA-map® SAPE, and GA-map® dH2O
- External Reagents: GA-map® Hybridization Buffer and GA-map® Detection Buffer
Storage: Box A is stored at -15°C, Box B is stored at 2-8°C, and the External Reagents are stored at 15-25°C
GA-map® Dysbiosis Test Lx Principle
The GA-map Dysbiosis Test Lx is a tool that allows mapping of the intestinal microbiota profile for a selected set of bacteria in a sample. The GA-map® Dysbiosis Test is based on advances in DNA profiling using probes targeting variable regions (V3 to V7) of the bacterial 16S rRNA gene to characterize and identify bacteria present. The targets are identified in a molecular multiplex assay that utilizes the Single Nucleotide Primer Extension (SNuPE) technology patented by professor Knut Rudi of the Norwegian University of Life Sciences (NMBU) (US6617138). A unique algorithm takes advantage of all the data generated by the detection of the SnuPE products to determine the microbiota profile in the sample. The algorithm is incorporated in the GA-map® Dysbiosis Analyzer software that accompanies the test.
Validated On:
- Luminex® 200™
- Luminex MAGPIX®
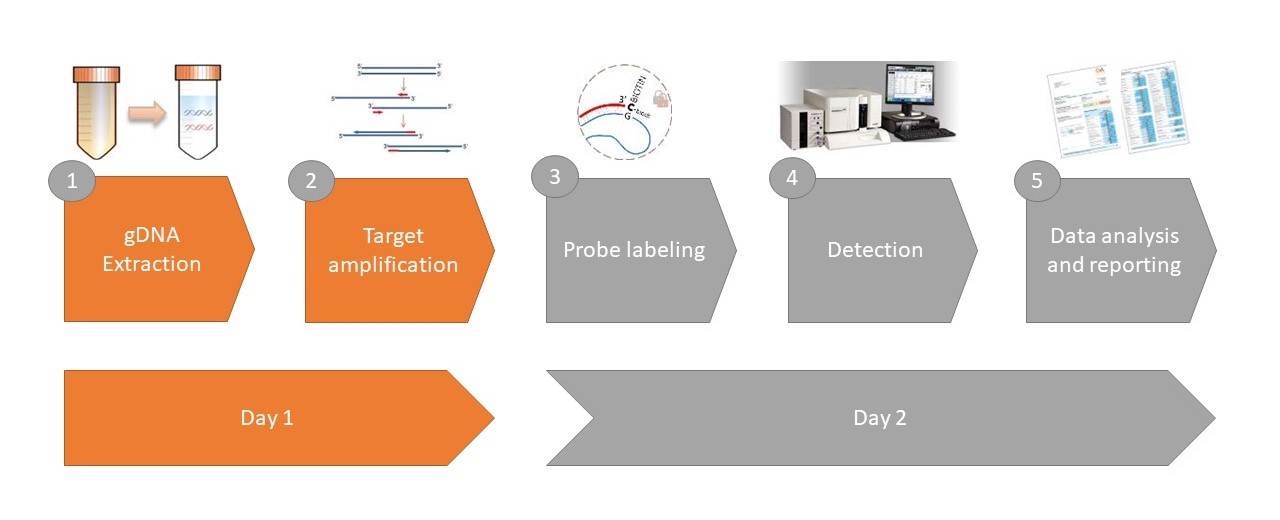
GA-map® Dysbiosis Test Lx Procedure Quick Guide
Related News
Eagle Biosciences Announces Distribution Agreement with Genetic Analysis
GA-Map Validated on MAGPIX
A Scientifically Validated Lab Test for Gut Dysbiosis


