AroCell AB (publ) announced today that a distribution
agreement has been signed with the New England-based company Eagle
Biosciences, for distribution of the AroCell TK 210 ELISA test in North
America.
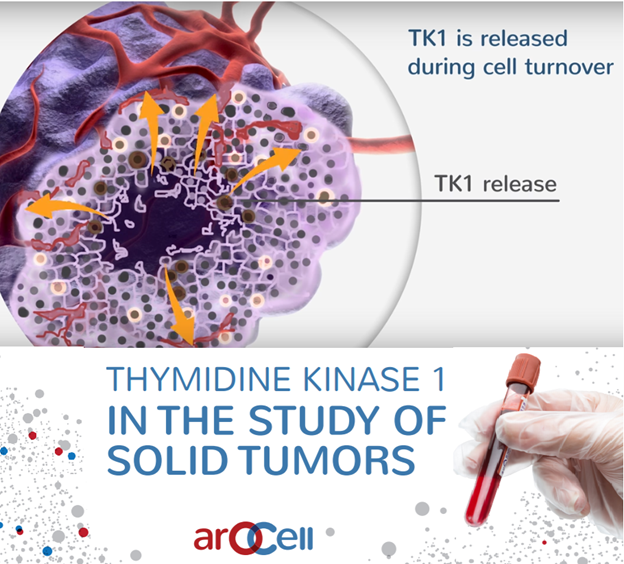
The TK 210 ELISA test measures TK1 protein levels for valuable
information about the speed of cell turnover. As tumors have high cell
turnover, the test may provide valuable information for prognosis and
optimization of treatment strategy.
“We are delighted to partner with Eagle Biosciences for the
distribution of the TK 210 ELISA test”, states Jan Stålemark, AroCell
CEO. “Eagle Biosciences is a leading provider of ELISA assay kits in
North America, and has primary focus on providing innovative products
that aid medical research and clinical laboratories. This highly concurs
with our strategy to make the TK 210 ELISA test widely available for
investigational use in the US, in order to facilitate use of the product
in cancer research institutions as well as within the pharmaceutical
industry”.
The TK 210 ELISA test will be commercially available immediately
through Eagle Biosciences for investigational use only in the United
States. The product can be found on the Eagle Biosciences website through the following link:
TK 210 ELISA
About AroCell
AroCell AB (AROC) is a Swedish company that develops
standardized modern blood tests to support the prognosis and follow up
of cancer patients. AroCell’s new technology is based on patented
methods to measure TK1 protein levels, which provide valuable
information about the speed of cell turnover. A tumor has high cell
turnover (speed of cell division and cell death) and as a result TK1 can
be detected in the blood with a simple laboratory test, called TK 210
ELISA. The test provides valuable clinical information for prognosis and
optimization of treatment strategy. The test may also be used for
monitoring disease relapse. For more information, please see www.arocell.com.
This information is information that AroCell is obliged to make public
pursuant to the EU Market Abuse Regulation. The information was
submitted for publication, through Jan Stålemark, at 11:45 CET on 6th October 2016. Redeye is AroCell:s Certified Adviser