Atezolizumab is a humanized monoclonal IgG antibody designed to target and bind programmed death-ligand 1 (PD-L1), thereby blocking its interaction with the programmed death receptor-1 (PD-1) and B7.1. This mechanism of action prevents the suppression of the anti-tumor immune response, allowing T cells to recognize and attack tumor cells more effectively. Unlike some other immune checkpoint inhibitors, atezolizumab does not rely on antibody-dependent cellular cytotoxicity, focusing instead on modulating immune checkpoint pathways.
Clinically, atezolizumab is approved for the treatment of several cancers, including locally advanced or metastatic urothelial carcinoma, particularly in patients ineligible for cisplatin-based chemotherapy or those who have progressed after platinum-containing regimens. It is also indicated as a first-line treatment in combination with bevacizumab, paclitaxel, and carboplatin for non-small cell lung cancer (NSCLC) in patients without EGFR or ALK mutations. Additionally, it is used in combination with paclitaxel protein-bound for treating PD-L1-expressing locally advanced or metastatic triple-negative breast cancer.
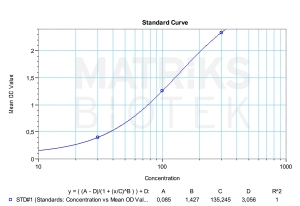
In research and therapeutic settings, Therapeutic Drug Monitoring (TDM) of atezolizumab is increasingly valuable for optimizing individual dosage regimens. Monitoring blood levels can help assess treatment efficacy, manage toxicity, avoid drug-drug interactions, and evaluate patient compliance. Like all biologics, atezolizumab’s immunogenicity profile may be influenced by manufacturing processes, and the potential formation of anti-drug antibodies (ADAs) can alter pharmacokinetics, reduce efficacy, or trigger adverse events. Understanding these dynamics is crucial for effective treatment planning and for evaluating biosimilar development.
This product is manufactured in Turkey by Matriks Biotek.