Palivizumab is a humanized monoclonal antibody (IgG1κ) that specifically targets the A antigenic site of the F protein of respiratory syncytial virus (RSV) subtypes A and B. Designed using recombinant DNA technology, palivizumab comprises 95% human and 5% murine antibody sequences. It is indicated for the prevention of serious lower respiratory tract infections in high-risk pediatric populations, particularly infants with prematurity, chronic lung disease, or congenital heart disease (CHD). By binding to the RSV F protein, palivizumab inhibits viral fusion and entry into host cells, thereby reducing the risk and severity of RSV infection.
In the clinical and research settings, palivizumab serves as a valuable biomarker tool for passive immunization strategies, especially in populations vulnerable to high RSV morbidity and mortality. Studies have shown that palivizumab administration significantly decreases RSV-related hospitalizations and ICU admissions among infants with CHD or other high-risk conditions. As such, it plays a vital role in disease prevention and outcome improvement in pediatric care.
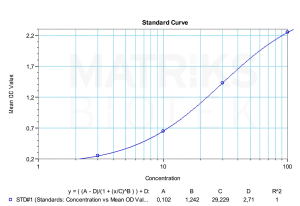
Furthermore, monitoring the immunogenic response to palivizumab is important in understanding its long-term efficacy and potential adverse effects. Like all biologic therapies, palivizumab can elicit antidrug antibodies (ADAs), which may alter drug pharmacokinetics or lead to reduced therapeutic benefit. Therefore, therapeutic drug monitoring (TDM) and ADA detection can enhance patient management, dosing accuracy, and overall clinical success in both routine practice and investigational studies.
This product is manufactured in Turkey by Matriks Biotek.