Infliximab is a monoclonal antibody that targets tumor necrosis factor alpha (TNFα), a central cytokine in chronic inflammatory diseases. By binding to both soluble and membrane-bound forms of TNFα, it blocks pro-inflammatory signaling, reduces immune cell migration, and lowers production of inflammatory cytokines and tissue-degrading enzymes. This action helps control inflammation across multiple cell types, including fibroblasts, endothelial cells, and lymphocytes, making infliximab effective in treating autoimmune and inflammatory conditions.
To optimize treatment, therapeutic drug monitoring (TDM) is used to maintain stable drug levels, improve efficacy, and minimize risks such as drug-drug interactions and toxicity. With the expiration of patents for first-generation biologics, biosimilars—highly similar but not identical biologic medicines—are now being developed and approved. Because of their complex manufacturing processes, exact copies cannot be made, and small variations may affect immune responses, potentially leading to reduced effectiveness or adverse effects. This highlights the importance of careful monitoring and evaluation when using both biologics and biosimilars.
This product is manufactured in Turkey by Matriks Biotek.
| Size | 1 x 96 Well |
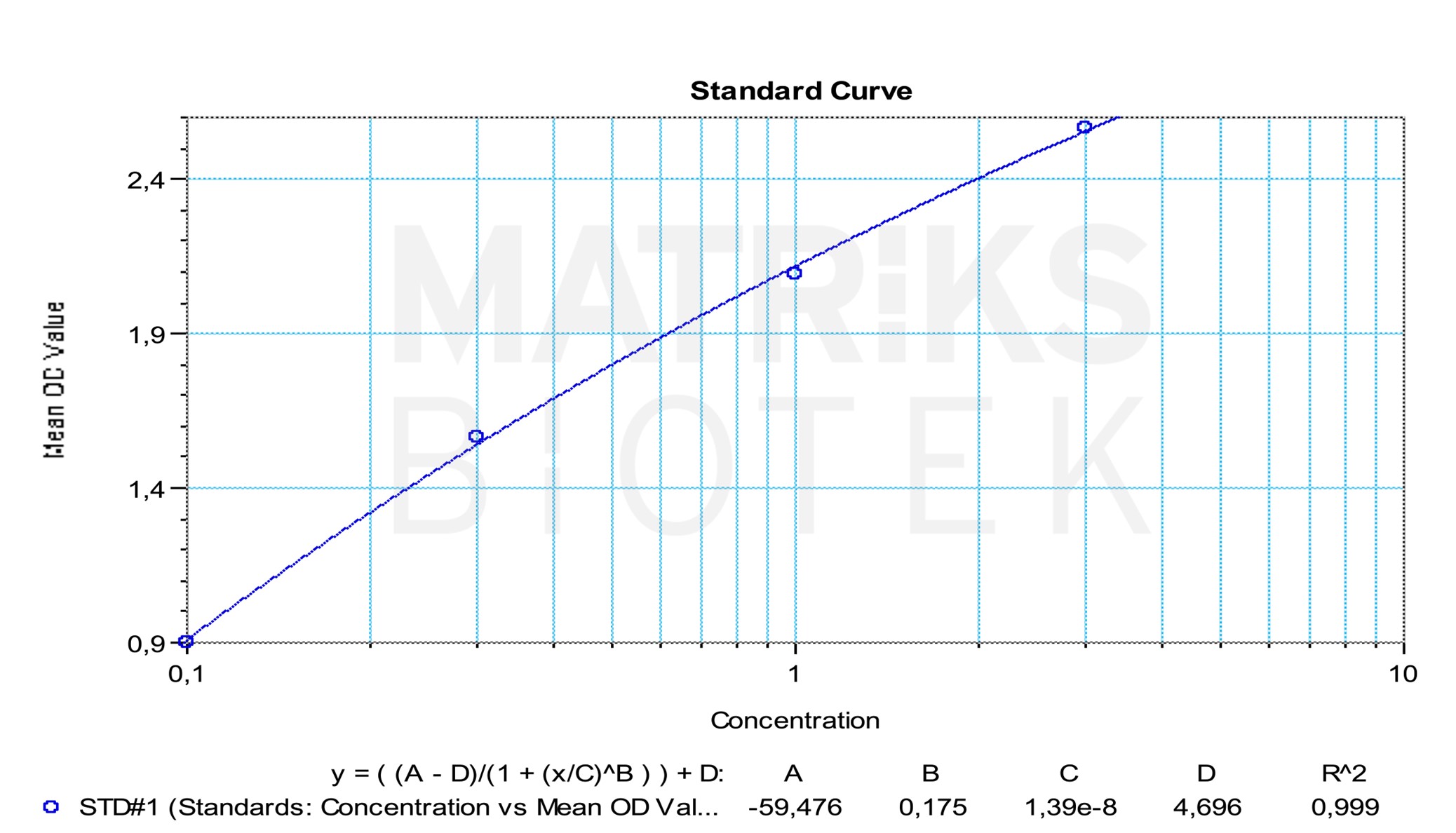
| Sensitivity | 0.1 µg/mL |
| Dynamic Range | 0.1 – 3 µg/mL |
| Incubation Time | 70 minutes |
| Sample Type | Serum, Plasma |
| Storage | 2-8°C |