Denosumab is a fully human IgG2 monoclonal antibody that specifically targets the receptor activator of nuclear factor kappa-B ligand (RANKL). RANKL is a critical regulator of bone remodeling, acting as the primary signal for osteoclast formation and bone resorption. Denosumab binds to RANKL with high affinity, preventing it from interacting with its receptor RANK on osteoclast surfaces, thereby inhibiting osteoclast-mediated bone destruction. This mechanism underlies its clinical utility in preventing bone loss in conditions such as metastatic bone disease and osteoporosis.
Clinically, denosumab is used to suppress bone resorption markers in patients with various skeletal-related disorders, particularly in those with bone metastases from solid tumors or multiple myeloma. By inhibiting the RANK/RANKL interaction, denosumab reduces osteoclast function and survival, leading to increased bone mass and strength in both cortical and trabecular bone. It is administered via subcutaneous injection at intervals of 1 to 4 weeks, depending on the clinical indication.
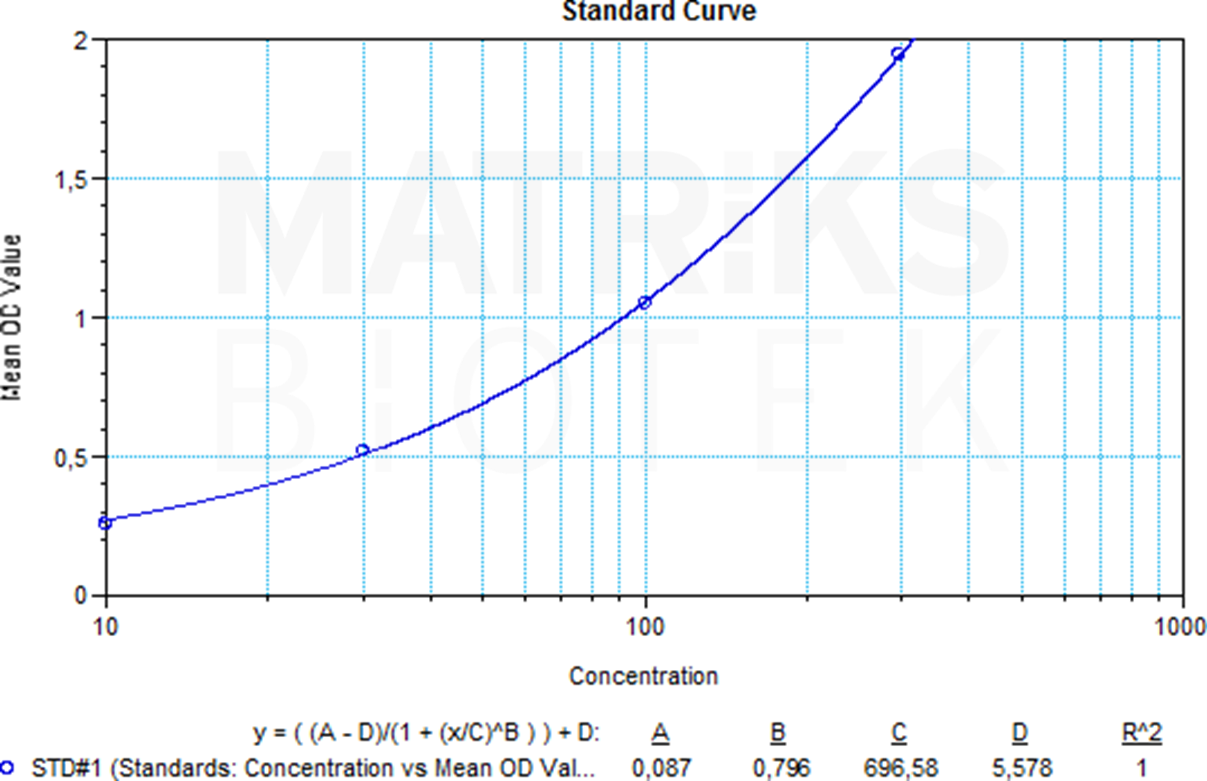
In research and therapeutic drug monitoring (TDM) settings, denosumab’s pharmacokinetics and immunogenicity are of particular interest. As a biologic, it may induce anti-drug antibodies (ADAs), which could affect its efficacy or safety. TDM helps assess drug levels, efficacy, and potential immune responses. Understanding the biomarker profile of denosumab and monitoring bone turnover markers in conjunction with RANKL levels allows for optimization of treatment strategies and evaluation of therapeutic responses in both research and clinical applications.
This product is manufactured in Turkey by Matriks Biotek.