Certolizumab pegol is a pegylated monoclonal antibody that targets tumor necrosis factor-alpha (TNF-α), a key pro-inflammatory cytokine involved in autoimmune and inflammatory conditions. Structurally, it is composed of a humanized Fab fragment (50 kDa) from an IgG1 isotype, conjugated to a 40 kDa polyethylene glycol (PEG) moiety that replaces the Fc region. The absence of the Fc region prevents complement fixation and antibody-dependent cytotoxicity, significantly enhancing the molecule’s half-life and reducing its immunogenic potential.
Certolizumab is particularly useful in clinical and research settings for its anti-TNF activity in conditions such as rheumatoid arthritis, Crohn’s disease, and other chronic inflammatory disorders. Its PEGylation and lack of glycosylation allow for efficient bacterial production, typically in E. coli, making it a cost-effective alternative to other TNF-α inhibitors. The drug’s design also allows for reduced immune-mediated side effects and more predictable pharmacokinetics.
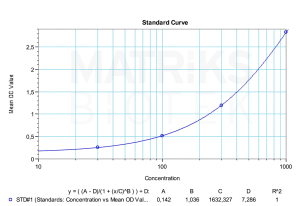
Therapeutic drug monitoring (TDM) of certolizumab pegol is critical to ensure optimal dosing, especially in patients with variable drug clearance or suspected immunogenicity. Monitoring for anti-drug antibodies (ADAs) can aid in identifying reduced drug efficacy or the need for alternative treatments. Given its unique structure and clinical advantages, certolizumab remains a valuable tool in both research studies and patient management for inflammatory and autoimmune diseases.
This product is manufactured in Turkey by Matriks Biotek.