Avelumab is a fully human monoclonal IgG1 lambda antibody that targets programmed death-ligand 1 (PD-L1), a key immune checkpoint protein expressed on tumor cells and immune cells within the tumor microenvironment. By binding to PD-L1 through its FG loops, avelumab blocks the interaction between PD-L1 and its receptors PD-1 and B7.1. This blockade removes the inhibitory signals on T cells, thereby restoring anti-tumor immune responses, including T-cell proliferation, cytokine release, and cytotoxic activity.
Unlike some other immune checkpoint inhibitors, avelumab also induces antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro, adding a direct tumor-killing mechanism in addition to immune modulation. Preclinical studies in syngeneic mouse tumor models have shown that blocking PD-L1 with avelumab results in decreased tumor growth, supporting its role in cancer immunotherapy.
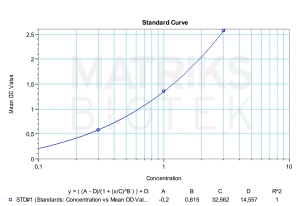
In research and clinical practice, therapeutic drug monitoring (TDM) of avelumab is useful for maintaining optimal drug levels in patients, especially in long-term or combination therapy settings. Monitoring helps optimize efficacy, manage adverse effects, and detect anti-drug antibodies (ADAs) that may reduce drug effectiveness or alter pharmacokinetics. As a biologic product, avelumab’s production involves complex, cell-based systems, and its immunogenicity profile may vary with even minor manufacturing differences—highlighting the importance of consistency in formulation and the challenges in biosimilar development.
This product is manufactured in Turkey by Matriks Biotek.