Infliximab is a chimeric monoclonal antibody that blocks tumor necrosis factor alpha (TNFα), a key cytokine in chronic inflammatory diseases. By binding to both soluble and transmembrane forms of TNFα, infliximab disrupts inflammatory signaling, reduces cytokine production, and limits immune cell migration to inflamed tissues. This results in decreased inflammation, reduced tissue damage, and lowered activity of enzymes that degrade joint and connective tissues. Its effects have been demonstrated across various human cell types, including fibroblasts, lymphocytes, and epithelial cells.
Because biologics like infliximab are structurally complex proteins produced using recombinant systems, therapeutic drug monitoring (TDM) is often employed to maintain effective and safe drug concentrations in patients. With the expiration of exclusivity periods for early biologics, biosimilar versions are being developed. However, exact copies cannot be made due to differences in host cells and manufacturing conditions. Even minor variations can alter immunogenicity, potentially leading to immune responses, reduced efficacy, or adverse events. As such, careful evaluation of biosimilars and monitoring of patient response remain essential.
This product is manufactured in Turkey by Matriks Biotek.
| Size | 1 x 96 Well |
| Sensitivity | 3.125 ng/mL |
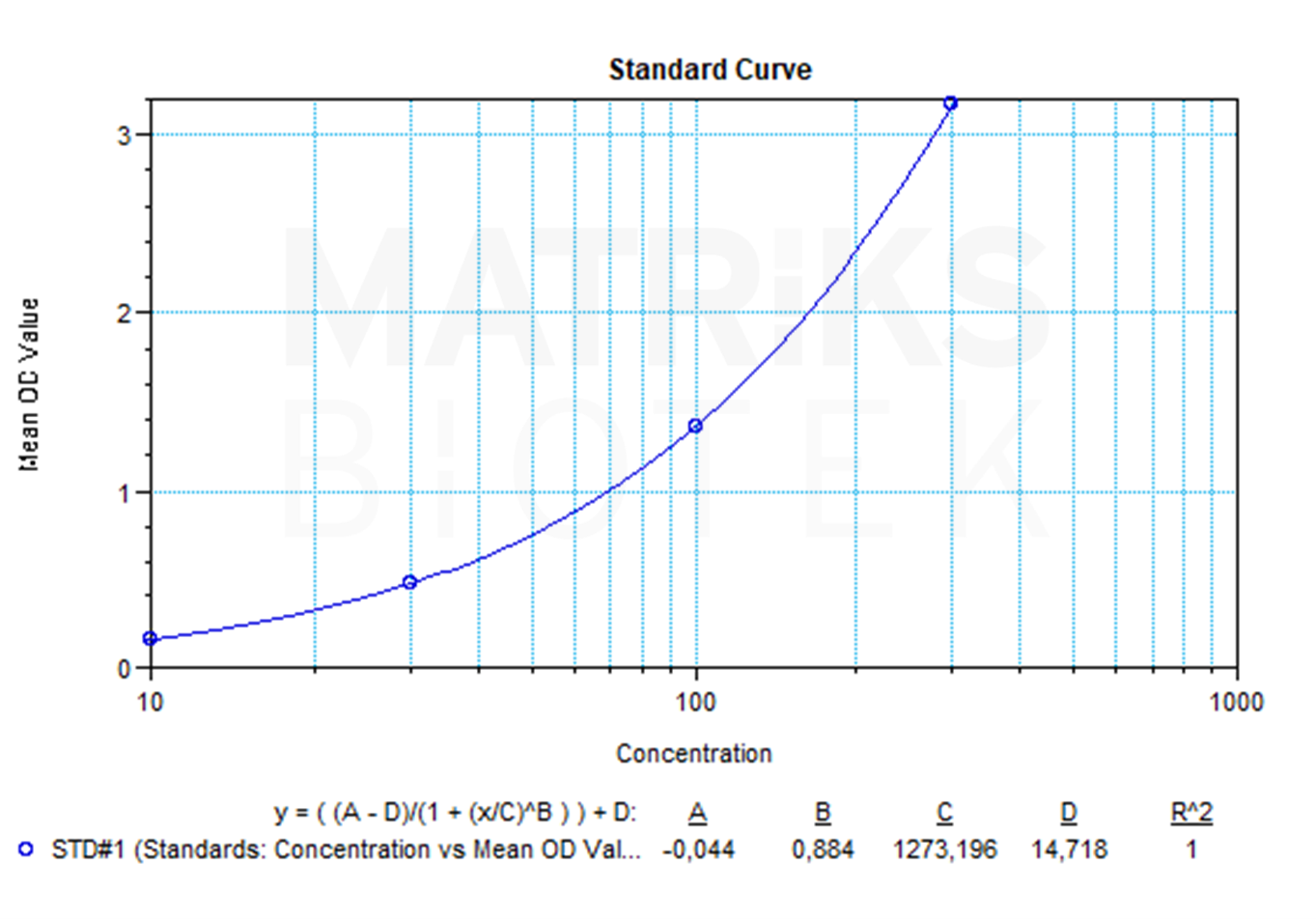
| Dynamic Range | 10-300 ng/mL |
| Incubation Time | 140 minutes |
| Sample Type | Serum, Plasma |
| Storage | 2-8°C |