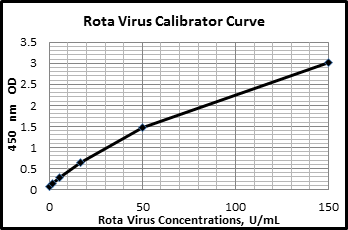
Rotavirus is a highly contagious virus and a leading cause of severe diarrheal illness in infants and young children globally. Detection of rotavirus in feces serves as a reliable biomarker for active infection, as the virus is shed in large quantities during acute gastrointestinal illness. As a biomarker, fecal rotavirus provides a direct measure of viral presence, shedding patterns, and infectious load, making it valuable for both surveillance and individual diagnosis. In research settings, fecal rotavirus biomarkers are used to evaluate vaccine efficacy, monitor outbreak dynamics, and study viral evolution and strain diversity. Longitudinal studies often rely on stool sampling to assess vaccine-derived immunity and the impact of interventions like sanitation improvements. Moreover, quantifying viral load in feces can inform disease severity and prognosis. With increasing global interest in rotavirus control and elimination, fecal biomarker analysis remains central to both epidemiological research and day-to-day clinical decision-making.
In research settings, Fecal Rotavirus Antigens Assay Kit Assay Kit is commonly used to investigate disease mechanisms, therapeutic targets, or diagnostic potential. Clinically, it may serve as a potential biomarker for disease detection, progression, or response to treatment depending on the context.
This product is manufactured in USA by Eagle Biosciences.
| Size | 1 x 96 Well |
| Sensitivity | Cut-off |
| Dynamic Range | Cut-off |
| Incubation Time | 2 hours |
| Sample Type | Stool |
| Storage | 2-8°C |
| Alternative Names | VP6, RV, Rotovirus Ag |