Rituximab is a chimeric monoclonal antibody that targets the CD20 antigen, a surface protein expressed on pre-B and mature B-lymphocytes. It functions by mediating B-cell lysis through mechanisms such as complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). The antibody belongs to the IgG1 subclass and includes a murine variable (Fab) region, responsible for antigen binding, and a human constant (Fc) region that initiates immune effector functions. This dual-region design allows rituximab to selectively bind to CD20 on B cells and recruit immune mechanisms for targeted cell destruction.
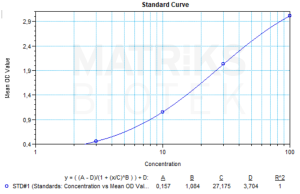
In clinical and research settings, rituximab is widely used as a biomarker-specific therapeutic agent in the treatment of hematologic malignancies and autoimmune disorders. Its specificity for CD20 enables targeted depletion of B cells, making it valuable in conditions such as non-Hodgkin lymphoma, chronic lymphocytic leukemia, and certain autoimmune diseases. The drug’s effectiveness can be monitored through therapeutic drug monitoring (TDM), which ensures optimal serum concentrations for therapeutic efficacy while minimizing toxicity and immune-related adverse effects.
Due to its biologic nature, rituximab has complex immunogenicity considerations. Anti-drug antibodies (ADAs) may form in response to treatment, potentially affecting pharmacokinetics, therapeutic outcomes, or safety profiles. As biosimilar versions of rituximab emerge, rigorous clinical and regulatory standards ensure that these products maintain equivalent safety, efficacy, and immunogenicity profiles. This makes rituximab not only a potent therapeutic tool but also a critical biomarker reference in immuno-oncology and immunology research.
This product is manufactured in Turkey by Matriks Biotek.