Ustekinumab is a fully human monoclonal IgG1 kappa antibody that targets the p40 subunit shared by interleukin-12 (IL-12) and interleukin-23 (IL-23), two key cytokines involved in inflammatory and autoimmune responses. By binding to the D1 domain of the p40 subunit in a 1:1 ratio, ustekinumab prevents IL-12 and IL-23 from interacting with their receptor complexes (IL-12Rβ1/2 and IL-12Rβ1/23R), thereby inhibiting downstream signaling in natural killer (NK) and T cells. This blockade results in suppression of Th1 and Th17 cell activation and limits the release of pro-inflammatory cytokines and chemokines.
Clinically, ustekinumab is categorized as a biologic disease-modifying anti-rheumatic drug (bDMARD) and is used to manage chronic inflammatory conditions such as psoriasis, inflammatory bowel diseases (IBD), and other autoimmune disorders involving dysregulation of IL-12/23 pathways. It reduces T-helper cell differentiation and cytokine production, improving patient outcomes in diseases with elevated IL-12/23 activity. Due to its selectivity for unbound IL-12 and IL-23, ustekinumab does not initiate Fc-mediated immune responses such as antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), enhancing its safety profile.
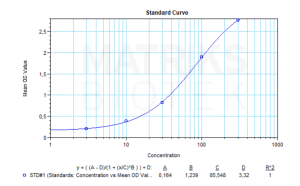
In a research or clinical setting, monitoring the pharmacokinetics and immunogenicity of ustekinumab is critical, particularly with therapeutic drug monitoring (TDM) to ensure optimal dosing and patient response. As with all biologics, ustekinumab may trigger anti-drug antibody (ADA) formation, which can affect drug efficacy and safety. Studies on its biosimilars, immunogenic profile, and ADA development remain essential for advancing personalized treatment strategies in immunology and rheumatology.
This product is manufactured in Turkey by Matriks Biotek.