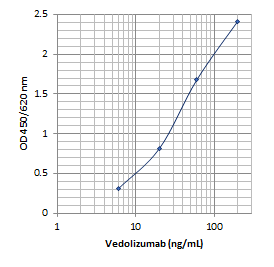
Vedolizumab mAb-based ELISA Assay
Vedolizumab mAb-based ELISA Assay is for Research Use Only
Size: 1 x 96 wells
Sensitivity: 5 ng/mL
Incubation Time: < 2 hours
Sample Type: Serum, Plasma
Sample Size: 10 µL
Alternative Names: Entyvio Assay
Assay Background
The drug Vedolizumab (trade name Entyvio®) is a humanized immunoglobulin G1 monoclonal antibody that binds exclusively to the lymphocyte integrin α4β7. Identification of biomarkers might be beneficial for (non-) response and risk factors for adverse drug reactions that might be related to serum drug levels and maintaining the effective concentration in order to potentially avoid some side effects with a reliable method. The specificity of this test system is achieved by using a monoclonal antibody named “IG-19F3” for the coating of the microtiter plate. This antibody is specific for Vedolizumab only and does not cross react with other lymphocyte integrin α4β7 catchers.
STORAGE AND STABILITY OF THE KIT
The kit is shipped at ambient temperature and should be stored at 2-8°C. Keep away from heat or direct sun light. The storage and stability of specimen and prepared reagents is stated in the corresponding chapters. The microtiter strips are stable up to the expiry date of the kit in the broken, but tightly closed bag when stored at 2–8°C.
The usual precautions for venipuncture should be observed. It is important to preserve the chemical integrity of a blood specimen from the moment it is collected until it is assayed. Do not use grossly hemolytic, icteric or grossly lipemic specimens. Samples appearing turbid should be centrifuged before testing to remove any particulate material.
Related Products
Vedolizumab (Entyvio®) Antibodies ELISA Kit
Infliximab (Remicade®) ELISA (mAb-based) Assay Kit
Total Antibodies to Infliximab (Remicade®) ELISA Assay