Infliximab is a monoclonal antibody that targets tumor necrosis factor alpha (TNFα), a key pro-inflammatory cytokine involved in chronic inflammatory diseases. By binding to both soluble and membrane-bound TNFα, it disrupts inflammatory signaling, reduces cytokine production, and limits immune cell migration to inflamed tissues. This mechanism decreases inflammation, induces apoptosis of TNF-producing cells, lowers endothelial adhesion molecules and acute phase proteins, and inhibits tissue-degrading enzymes, helping control inflammatory activity across multiple cell types.
To ensure optimal treatment, therapeutic drug monitoring (TDM) is used to maintain steady drug concentrations in the bloodstream. TDM supports dosage optimization, improves compliance, prevents drug interactions, and reduces toxicity risks. As patents for early biologics expire, biosimilars—products highly similar to reference biologics—are becoming available. However, because biologics are complex proteins produced in living systems, exact copies cannot be manufactured. Even small variations can alter immunogenicity, potentially triggering anti-drug antibodies (ADAs) that affect pharmacokinetics, reduce effectiveness, or cause adverse effects. Careful monitoring is therefore critical when using both biologics and biosimilars.
This product is manufactured in Turkey by Matriks Biotek.
| Size | 1 x 96 Well |
| Sensitivity | 6.25 ng/mL |
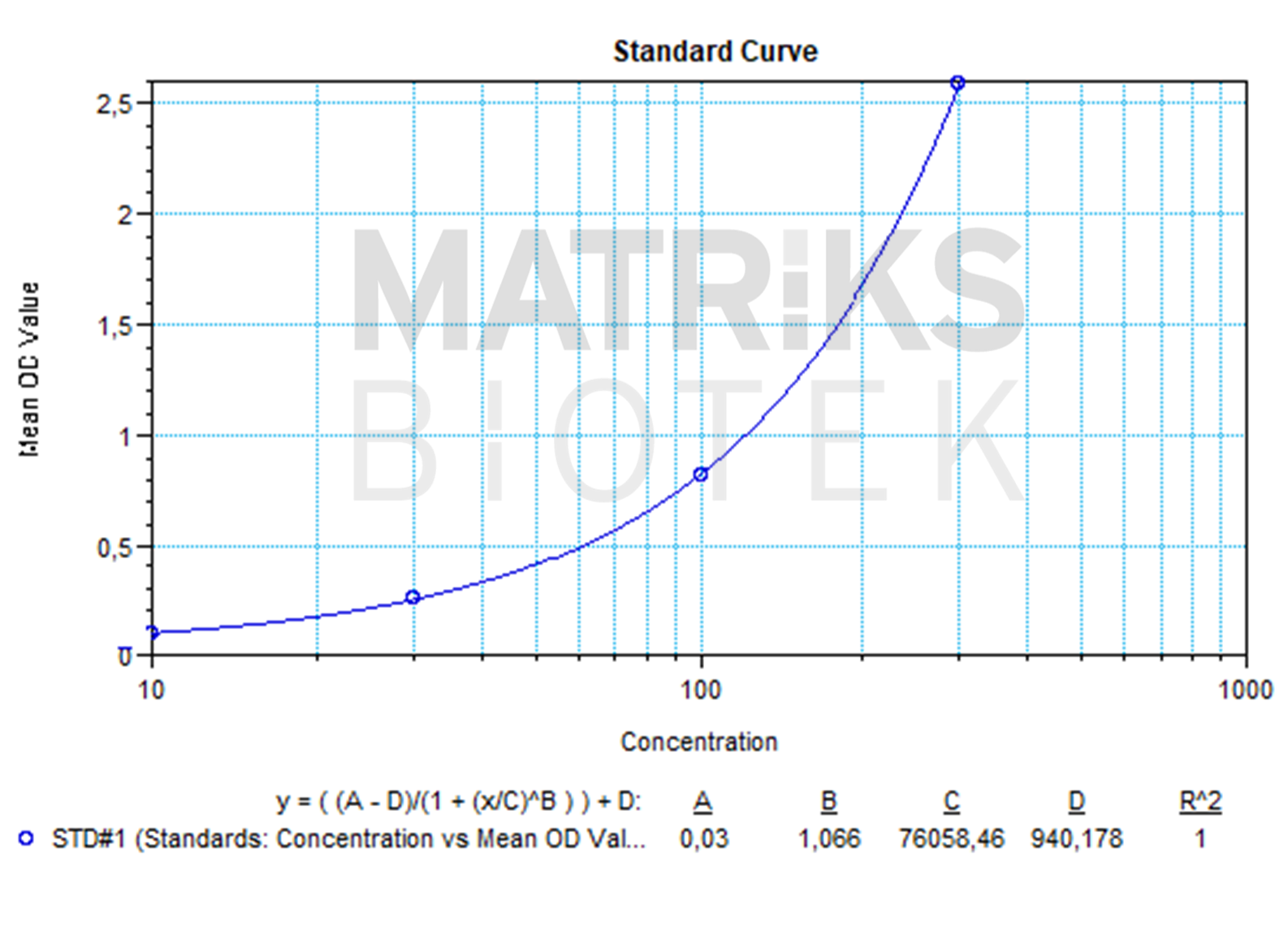
| Dynamic Range | 10-300 ng/mL |
| Incubation Time | 140 minutes |
| Sample Type | Serum, Plasma |
| Storage | 2-8°C |